Cancer of the Uterine Cervix
Our science focuses on early cervical cancer detection. Worldwide, cervical cancer is the fourth most common cancer in women, and the seventh overall, with an estimated 570,000 new cases in 2018 and about 311,000 women died from the disease. To reduce the morbidity and mortality from cervical cancer, cervical screening has been adopted in many countries. Early detection, when the cancer or its precursor lesion is still treatable and confined to the organ of origin, is one of the best ways to prevent and survive cervical cancer. However, even in countries with cervical screening programs, women are annually diagnosed with and die from cervical cancer.
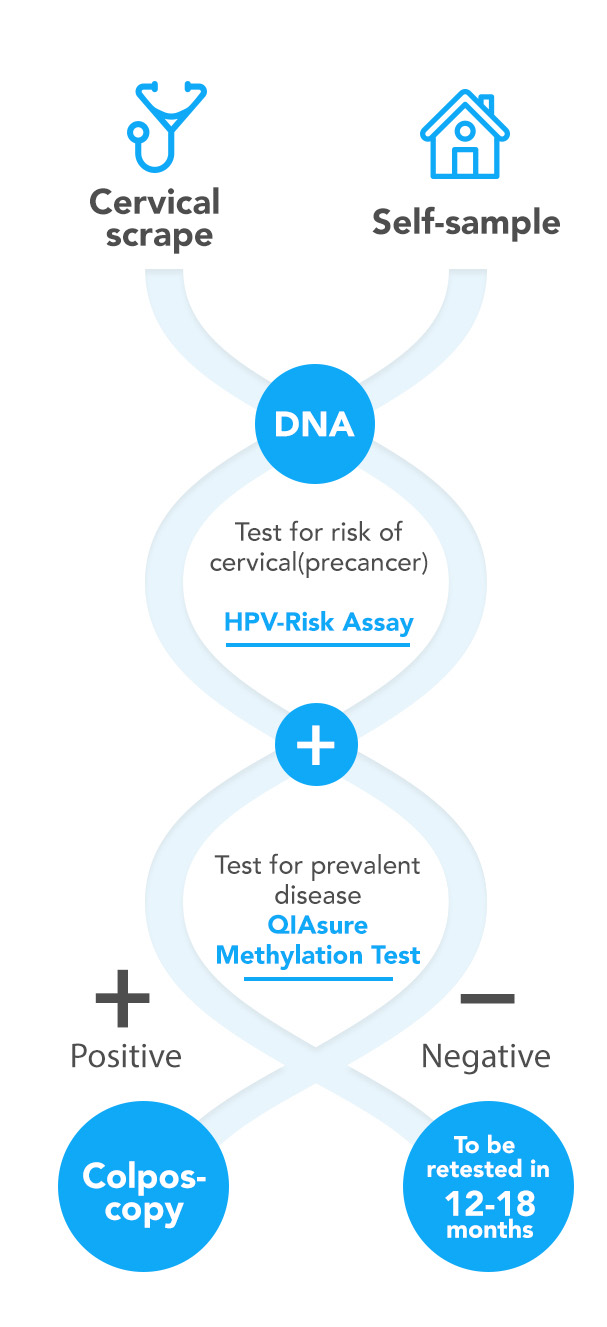
Reasons for cervical cancer incidence despite screening, include the use of an imperfect screening tool and non-compliance to screening. In many countries cytological examination of cervical smears is used as a primary or triage tool. Approximately 30% of the (pre)cancer conditions are not detected by cytological examination. Women who are never or under-screened have an increased risk of invasive cervical cancer. Self-sampling approaches have shown to increase the screening participation rate. Self-screen develops and validates its molecular assays to be compliant with self-collected specimens.
Human Papillomavirus (HPV)
Cervical cancer is caused by a persistent infection with high-risk human papillomavirus (hrHPV). Most infections are cleared by the woman’s immune system but some are persistent and may result in transforming lesions of the cervix (high-grade cervical intraepithelial neoplasia or transforming CIN) and, if left untreated, ultimately to the development of cervical cancer. A considerable improvement in the effectiveness of cervical cancer screening can be achieved by using a clinically-validated hrHPV DNA detection assay as a primary screening tool. The HPV-Risk Assay has been validated for screening purpose, and can be used to determine the presence of these clinically relevant amounts of HPV DNA in a cervical physician-taken or self-collected sample (ref 5).
Methylation markers for high-grade cervical disease
The fact that most hrHPV infections are transient and do not give rise to clinically meaningful disease asks for triage methods following primary hrHPV DNA testing. This is required to reduce unnecessary follow-up of women without clinically meaningful hrHPV infections. Self-screen’s research has focused on the identification of disease markers comprising specific (epi)genetic alterations in the host cell genome that are, in addition to a persistent hrHPV infection, required for the development of invasive cervical cancer. This work resulted in several patented methylation markers that enable triage testing of HPV-positive women to identify those with a high short-term risk for the development of cervical cancer and need to be referred for colposcopy (ref 1, 2, 3, 7, 8, 9, 10, 11).