Full-molecular automated cervical screening is now possible combining the objectivity, reproducibility and high-throughput aspects of both the HPV-Risk Assay and the QIAsure Methylation Test.
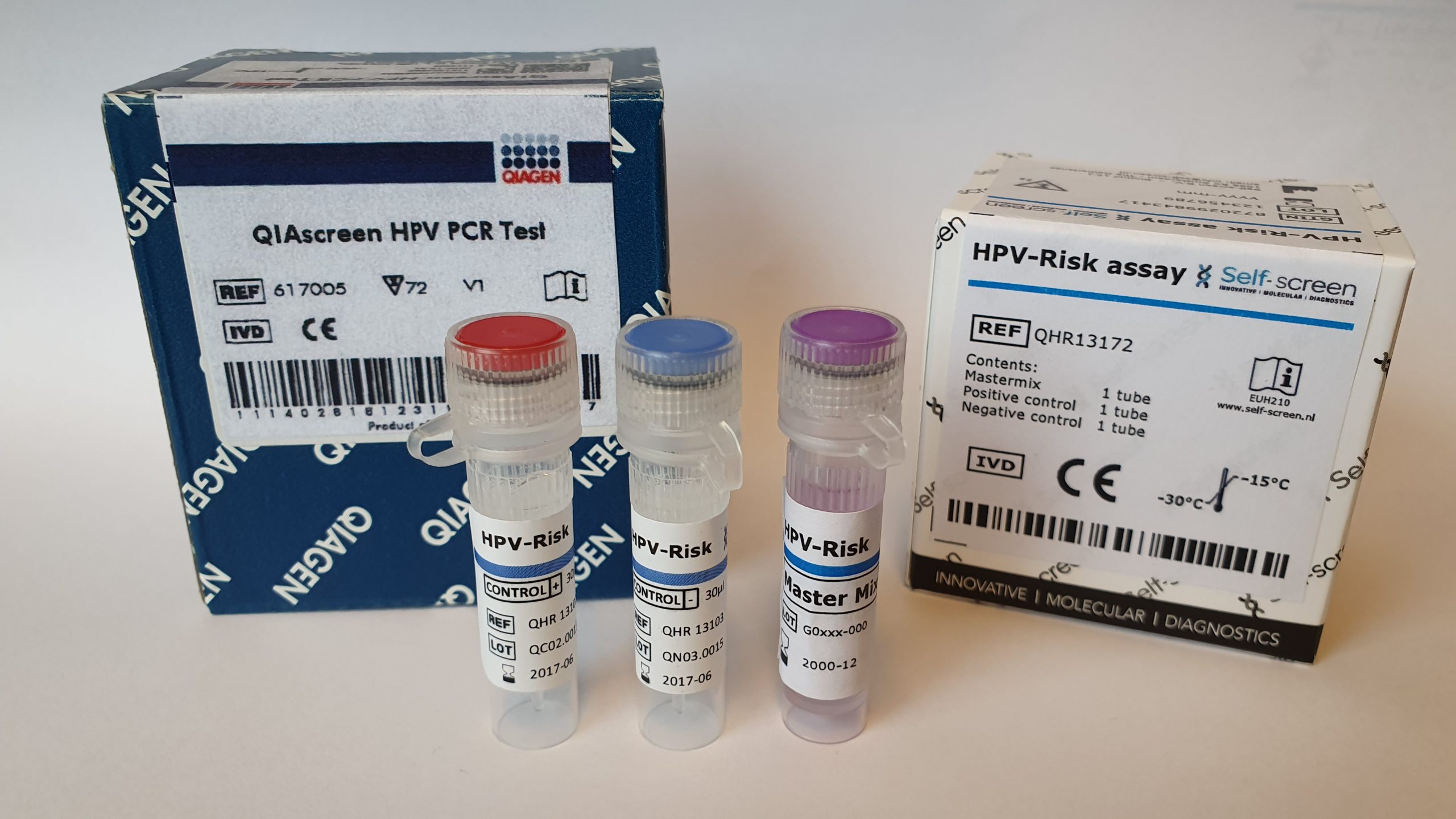
The HPV-Risk Assay is an in vitro real-time PCR-based assay for the qualitative detection of human papillomavirus (HPV) DNA, targeting the E7 region of the following 15 (probably) high-risk HPV genotypes, i.e., 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 67, and 68.
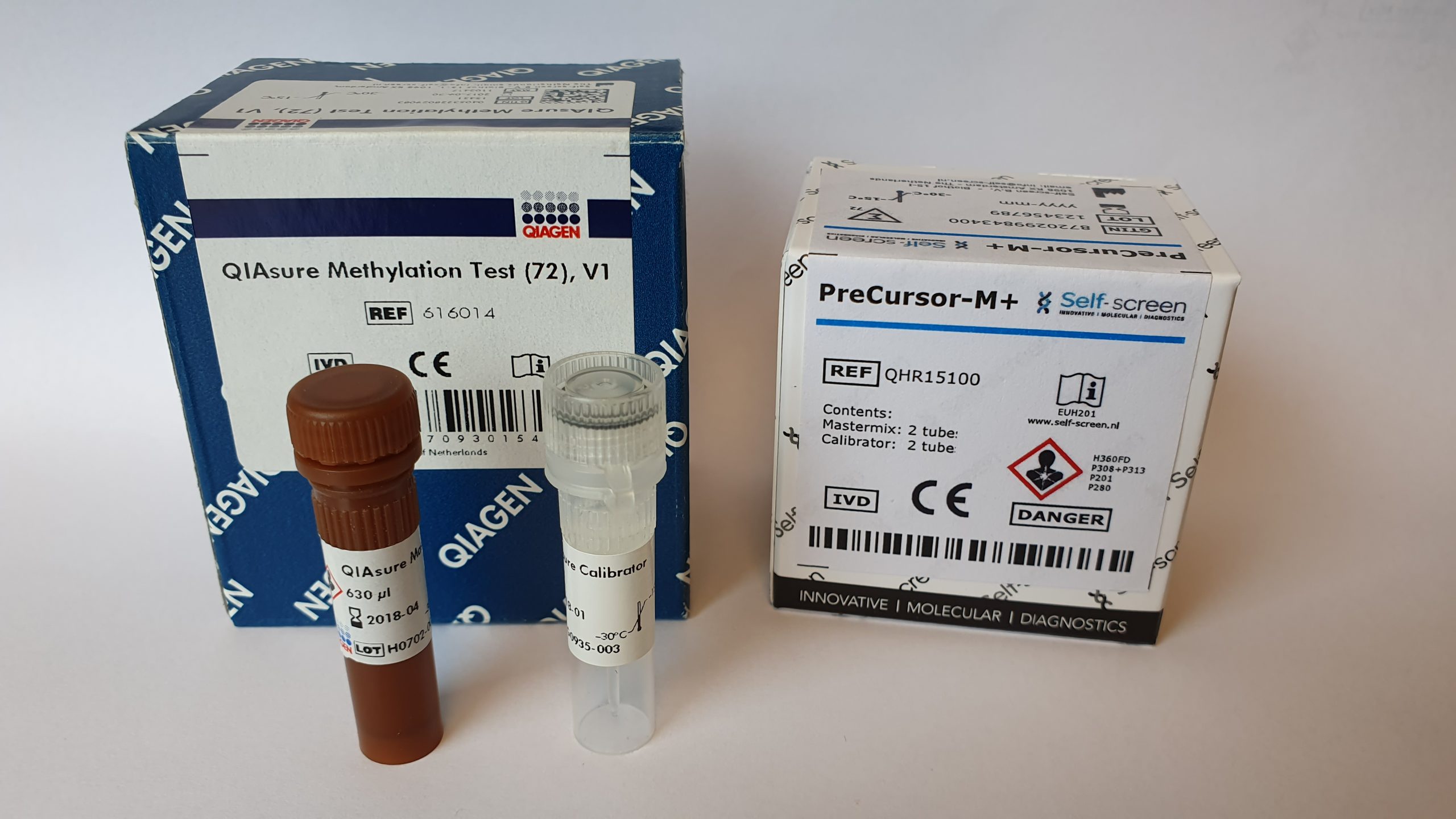
The QIAsure Methylation Test is a real-time PCR-based molecular assay to distinguish women with cervical (pre)cancer. The QIAsure Methylation Test specifically detects women with a cancer-like methylation profile that have a short term high risk for progression to cervical cancer.
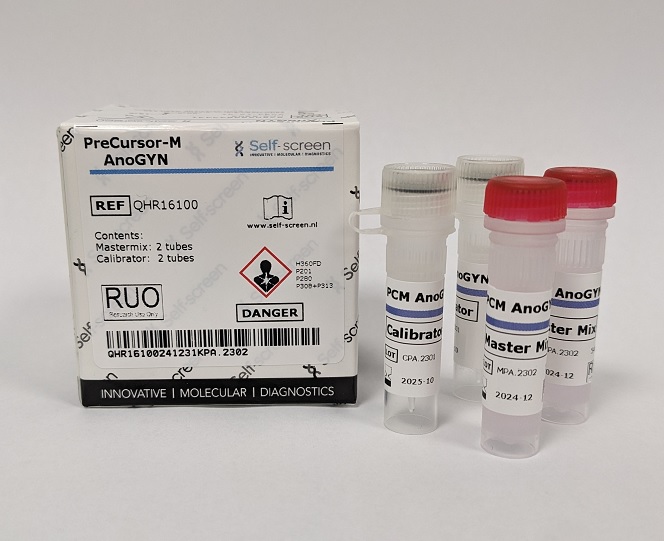
Self-screen has developed the PreCursor-M AnoGYN Methylation Assay (RUO). This assay identifies the hypermethylation levels of two genes, ASCL1 and ZNF582, respectively in anal specimens. With the ACTB reference gene, the PreCursor-M AnoGYN is developed as a multiplex quantitative methylation specific PCR on the RotorGeneQ (QIAGEN), Mic (Biomolecular Systems) and ViiA7 (ThermoFisher) cyclers.
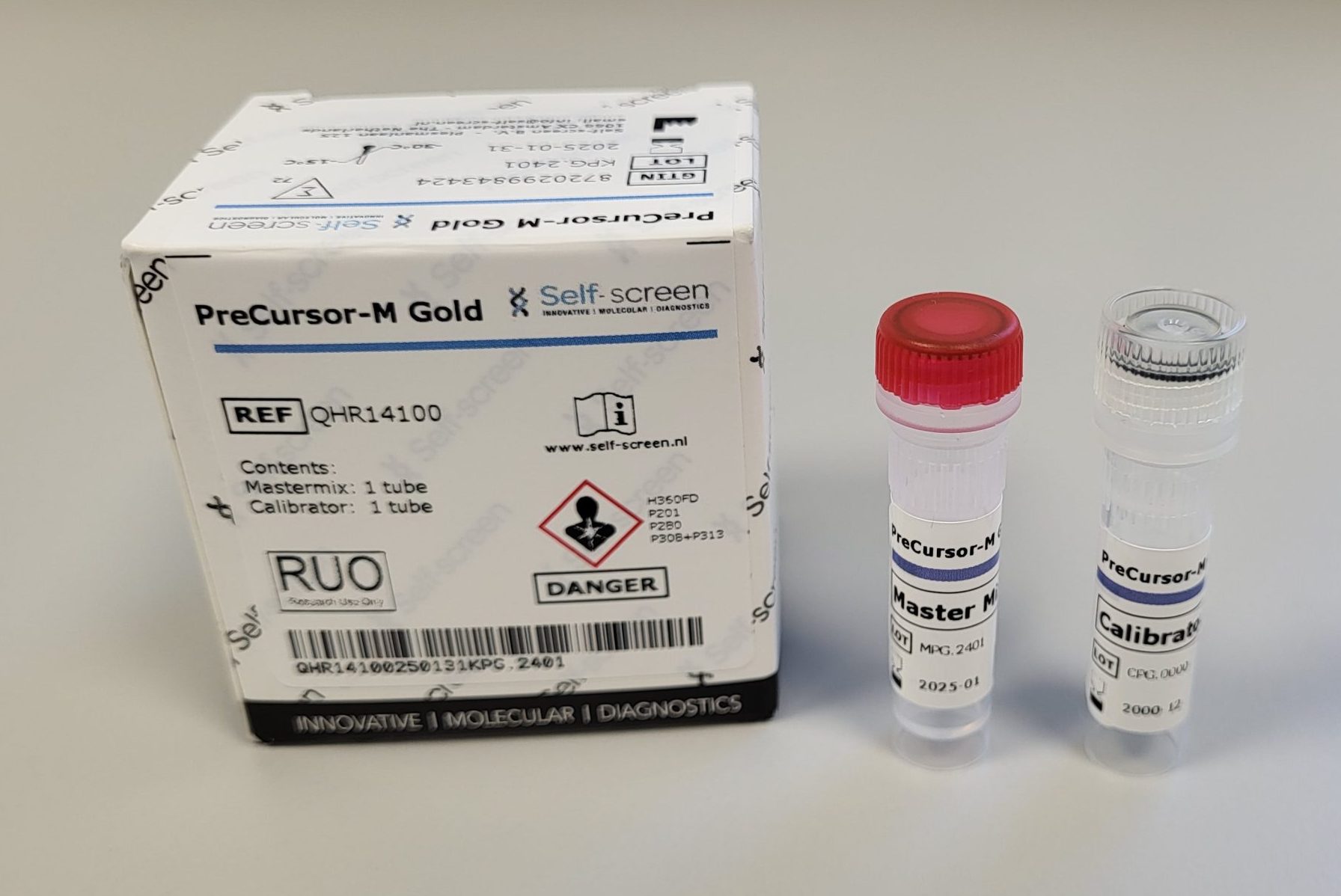
The methylation assay pipeline of Self-screen has now been extended with the Precursor-M Gold assay. The newly developed Precursor-M Gold assay is currently available for Research Use Only indications.
Precursor-M Gold demonstrates very good clinical performance in the detection of high grade precursor stadia and cervical cancer among HPV positive women. The assay is ready for further IVD validation on a platform or workflow of choice.
Our Methylated DNA Control Sample is an essential tool for researchers conducting bisulfite conversion and methylation-specific PCR (MSP) testing.
Incorporating the Methylated DNA Control Sample into your workflow supports consistent results, whether used regularly for quality control or as a troubleshooting aid when unforeseen results arise.
Ideal for use with our Self-screen Methylation-specific PCR kits (Precursor-M+, Precursor-M AnoGYN, Precursor-M Gold) as well as other MSP kits, this control provides confidence in your results—whether for routine quality control, periodic data monitoring, or troubleshooting.
Self-screen is constantly developing novel state-of-the-art clinical tests for (early) cancer detection. These tests meet growing requirements in both primary screening as well as triage settings.


