HPV Risk assay/ QIAscreen HPV PCR Test
HPV Risk assay for screening
The HPV-Risk assay (CE-IVD) is intended to be used for the screening of women at risk of cervical (pre)cancer.
The test is an in-vitro real-time PCR-based DNA assay for the qualitative detection of 15 (probably) high-risk human papillomavirus (HPV) genotypes: i.e. 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 67 and 68.
What makes the HPV-Risk assay unique?
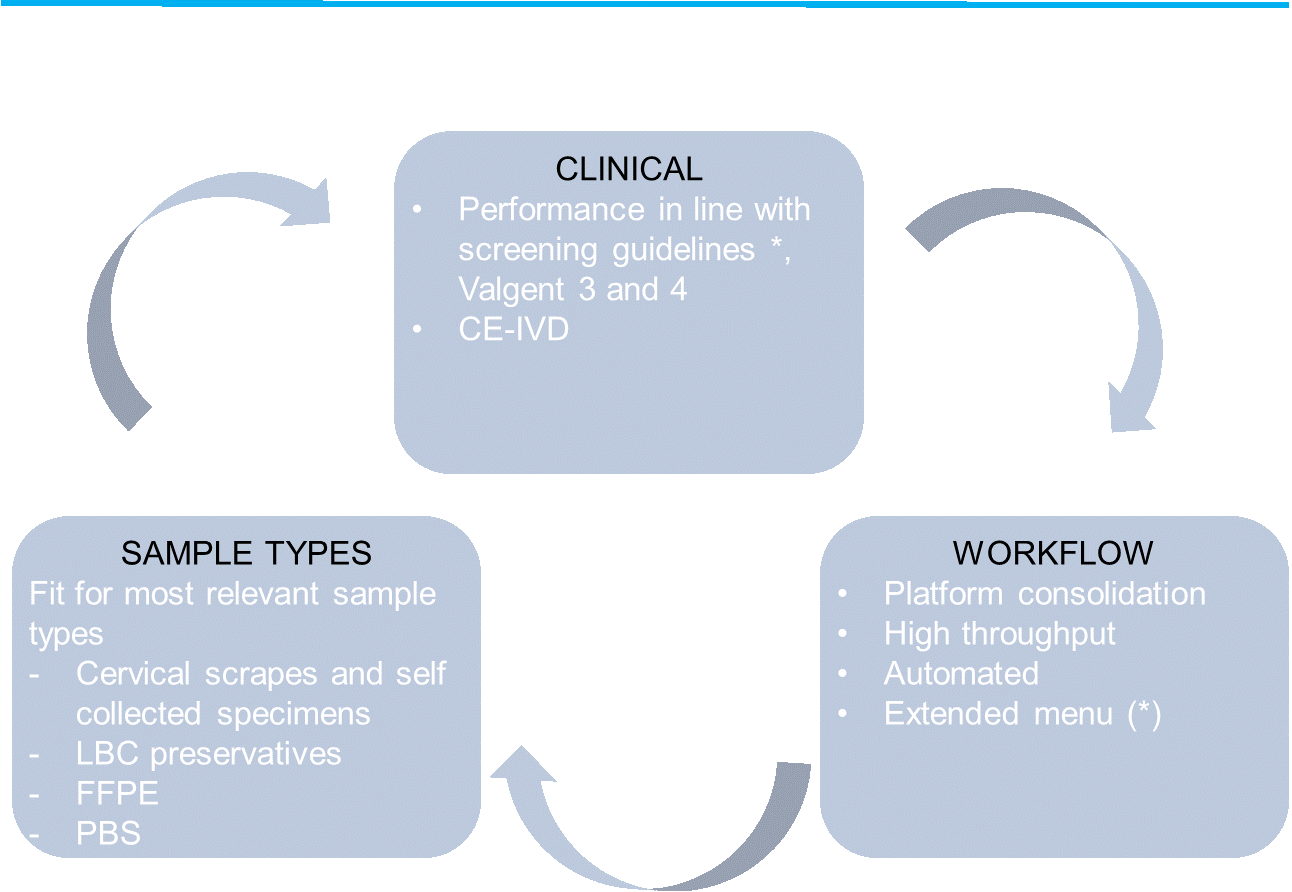
- The assays is clinically validated and demonstrates good clinical performance compliant to the International Guidelines (also known as the Meijer criteria) on clinical sensitivity, clinical specificity and intra and inter laboratory performance. The clinical sensitivity and specificity for CIN2+ were non-inferior to that of the reference assay GP5+/6+, indicating a very good clinical performance.
| Clinical sensitivity | Clinical specificity | |
|---|---|---|
| CIN2+ | 97.1% (67/69) | 94.3% (777/824) |
- The assay targets the E7 region of the HPV genome, a region which is always retained in cervical cancer. Thereby potential false-negative results due to the effects of viral integration, a process that is often associated with malignant progression, are prevented. As viral integration causes interruption of the viral genome in a region from the E1 to the L1 ORF it can only affect assays targeting within this region like L1-based PCR detection assays.
- The most abundant and carcinogenic genotypes HPV16 and HPV18 are reported separately from non-HPV 16/18 high risk types.
- An internal sample control checks for sample quality, assuring reliable results.
- Beyond cervical scrapes, it performs well on a large variety of different sample specimens. The assay is successful validated in the VALGENT-3 and VALGENT-4 studies against the reference assays GP5+6+ and hc2 for several preservatives such as PreservCyt and SurePath. In addition it is also validated for self-samples (brush and lavage) and can be used for detection of HPV in formalin fixed paraffin embedded (FFPE) specimens.
- The HPV Risk Assay is compatible with the following real time PCR platforms: RotorGeneQ and Mic qPCR Cycler, and Biorad CFX, ABI7500 and VIA7.
Workflows
DNA extraction for the HPV-Risk assay can be performed fully automated using the QIAsymphony® DSP Virus/Pathogen Midi Kit including PCR set up, or semi-automated using the NucleoMag 96 Tissue kit from Macherey-Nagel (can be performed on several 96 well liquid handlers), but also manually using standard DNA extraction kits (e.g., column- and magnetic bead-based kits, such as QIAamp® DSP virus spin kit).
HPV Risk assay literature and studies
Meijer et al. J. Cancer. 2009 February 1; 124(3): 516-520
About: What are the consensus requirements that an HPV screening assay should fulfill?
Clinical validation of the HPV-Risk Assay, a novel real-time PCR assay for detection of high-risk human papillomavirus DNA by targeting the E7 region.
Hesselink et al. J Clin Microbiol. 2014;52(3):890-896.
About: The clinical performance of the HPV-Risk Assay as HPV screenings assay, on clinician taken cervical scrapes and self-samples.
Evaluation of the Clinical Performance of the HPV-Risk Assay Using the VALGENT-3 Panel.
Polman et al, J Clin Microbiol. 2017
About: The clinical performance of the HPV-Risk Assay on PreservCyt samples compared to the HC2 reference HPV assay.
Heideman et al, J. Clin. Virol. 2019
About: The clinical performance of the HPV-Risk Assay on SurePath samples compared to the GP5+/6+-PCR-EIA reference HPV assay and GP5+/6+-PCR-EIA-LMNX.
Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening?
Arbyn et al. Clin Microb & Inf 2015
About: Review comparing clinically validated HPV assays, demonstrating good clinical performance of HPV-Risk Assay
For ordering and pricing information, please contact us at info@self-screen.nl